
How many valence electrons does neon(Ne) have?
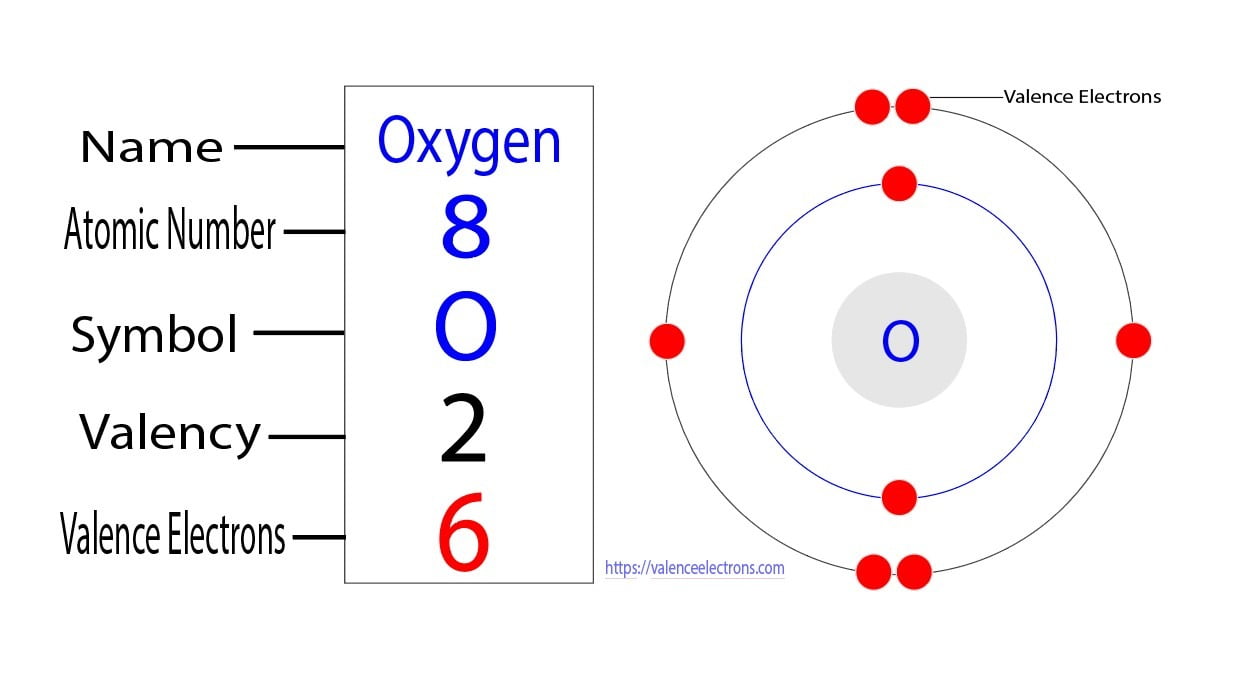
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan. Questions.
PPT Unit 3 PowerPoint Presentation, free download ID5685070
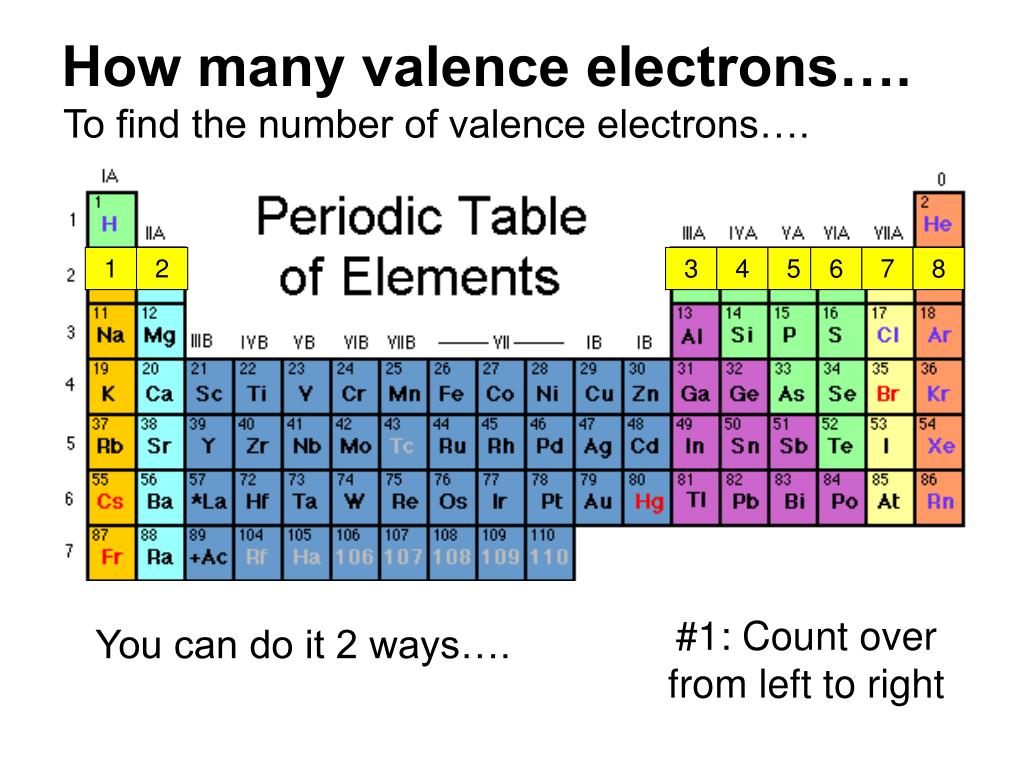
There are two ways to find the number of valence electrons in Neon (Ne). The first is to use the Periodic Table to figure out how many electrons Neon has in.

Free Download Periodic Table Help Names Valence Electrons For Free Uploadto
This table of element valences includes the maximum valence and most common valence values. Use this is a reference with a periodic table.. You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be. Neon: 0: 11: Sodium +1: 12: Magnesium +2: 13: Aluminum +3: 14.

Electron Configuration Chart With Orbitals
Four covalent bonds.Carbon has four valence electrons and here a valence of four. Each hydrogen atom has one valence electron and is univalent. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both.

Periodic table of elements with valence electrons bannerhome
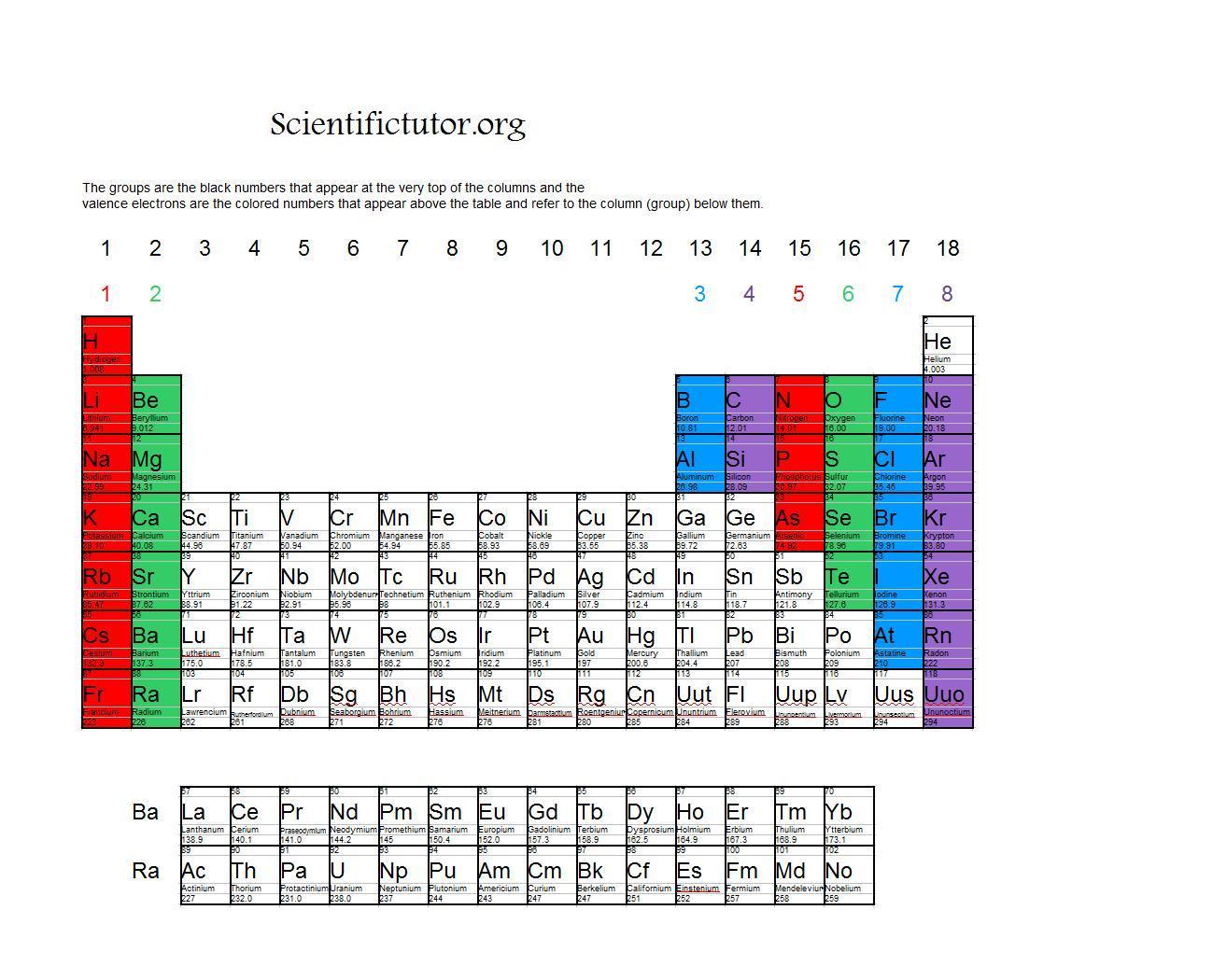
Each element has a number of valence electrons equal to its group number on the Periodic Table. Figure %: The periodicity of valence electrons This table. Helium (He) and Neon (Ne) have outer valence shells that are completely filled, so neither has a tendency to gain or lose electrons. Therefore, Helium and Neon, two of the so-called Noble.
Electron Dot Diagram Periodic Table
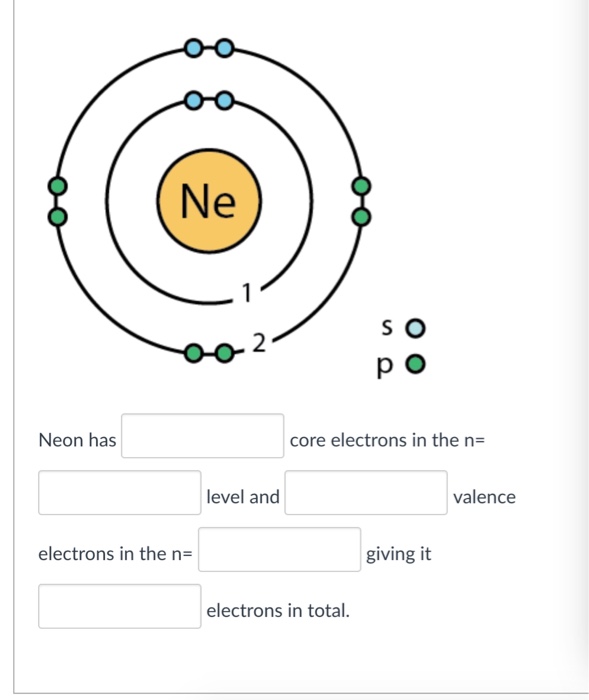
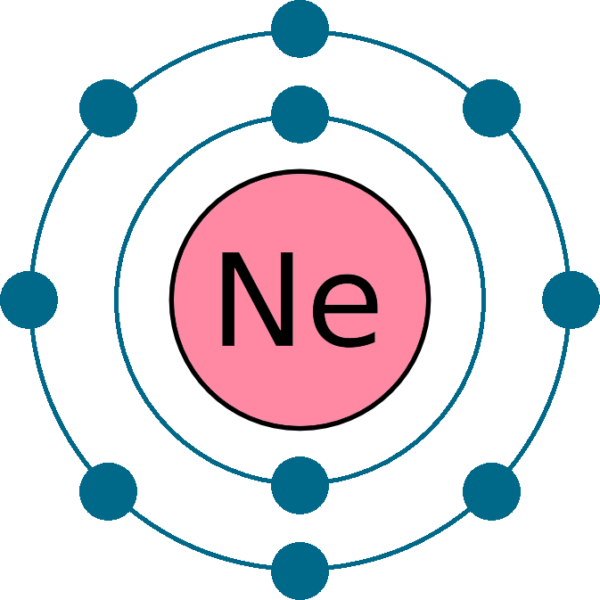
The electronic structure of neon (Ne) is 1s 2 2s 2 2p 6. This means that neon has two electrons in the 1s orbital, two electrons in the 2s orbital, and six electrons in the 2p orbitals. Neon has a completely filled valence shell, making it an inert or noble gas.
Solved Ne 2 p o Neon has core electrons in the n level and
And you have one more electron to worry about. And so that electron would go into a 3S orbital. So the full electron configuration is 1S2, 2S2, 2P6, and 3S1. When I want to figure out how many valence electrons sodium has, the number of valence electrons would be equal to the number of electrons in the outermost shell, the outermost energy level.

How to find Valency? What are valence electrons? Teachoo (2023)
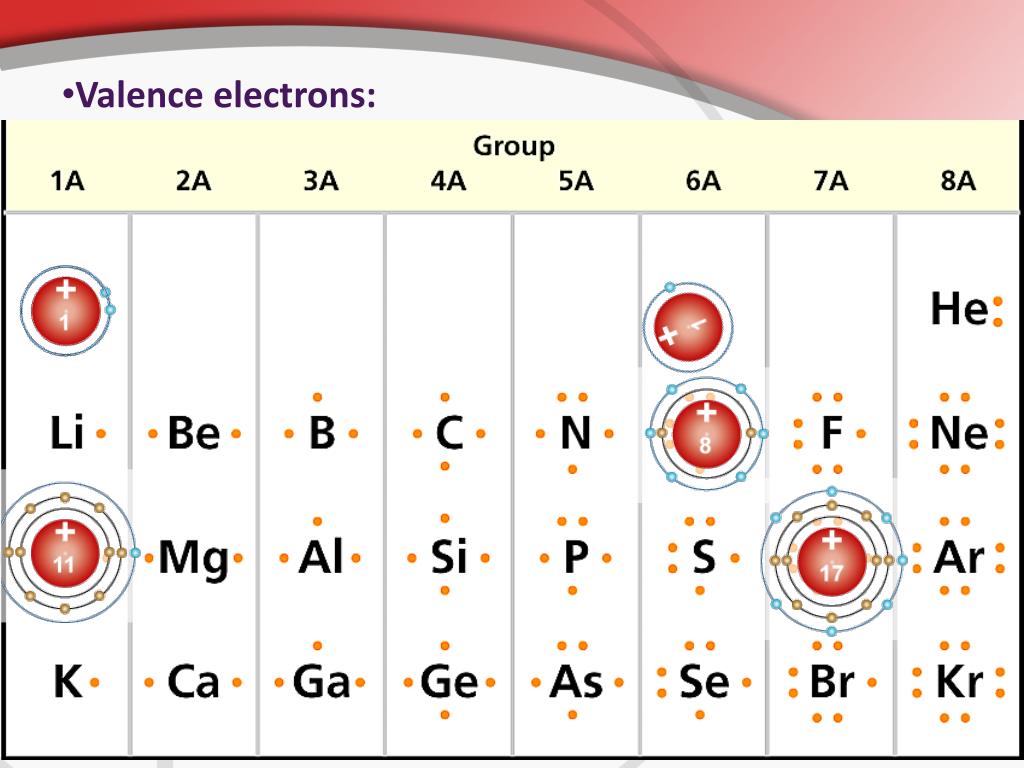
Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons.
What Are Valence Electrons And How To Find Them Where Are They Located
Explanation: Neon, Z = 10, has eight valence electrons. This closed shell configuration makes neon supremely difficult to oxidize, and difficult to reduce. The inertness, the lack of reactivity, of this Noble Gas, is a function of its electronic configuration. To which group of the Periodic Table does neon belong? Neon, Z=10, has eight valence.

Electron Configuration Of Neon In Excited State worksheet today
It simply depicts the theory that the first two orbital the 1s and the 2s are having 2 electrons respectively. The remaining 6 electrons have been held by the 2p orbital. How Many Valence Electrons Does Neon Have? Neon basically has the 8 valence electrons as its full octet and this is what makes neon stable in the terms of a noble gas.

How many valence electrons does neon(Ne) have?
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
Electron Configuration Of Neon My XXX Hot Girl
Step-3: Determine the valence shell and calculate the total electrons. The third step is to diagnose the valence shell (orbit). The last shell after the electron configuration is called the valence shell. The total number of electrons in a valence shell is called a valence electron. The electron configuration of neon shows that the last shell.
Valence Electrons In O2 Factory Clearance, Save 68 jlcatj.gob.mx
Created by video journalist Brady Haran working with chemists at The University of Nottingham. Element Neon (Ne), Group 18, Atomic Number 10, p-block, Mass 20.180. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
PPT Classic Chem PowerPoint Presentation, free download ID2464733
For example, silicon is in Group IVA (Group 14), so each atom would have four valence electrons. Chlorine is in Group VIIA (Group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in Group IIA (Group 2). Helium is the only exception for the main group elements. The first energy level holds a.
How Many Valence Electrons Are in the Neon Family
How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three.. Neon, with its configuration ending in \(2s^2 2p^6\), has eight valence electrons. Valence electrons for transition elements. Transition elements are a bit trickier.

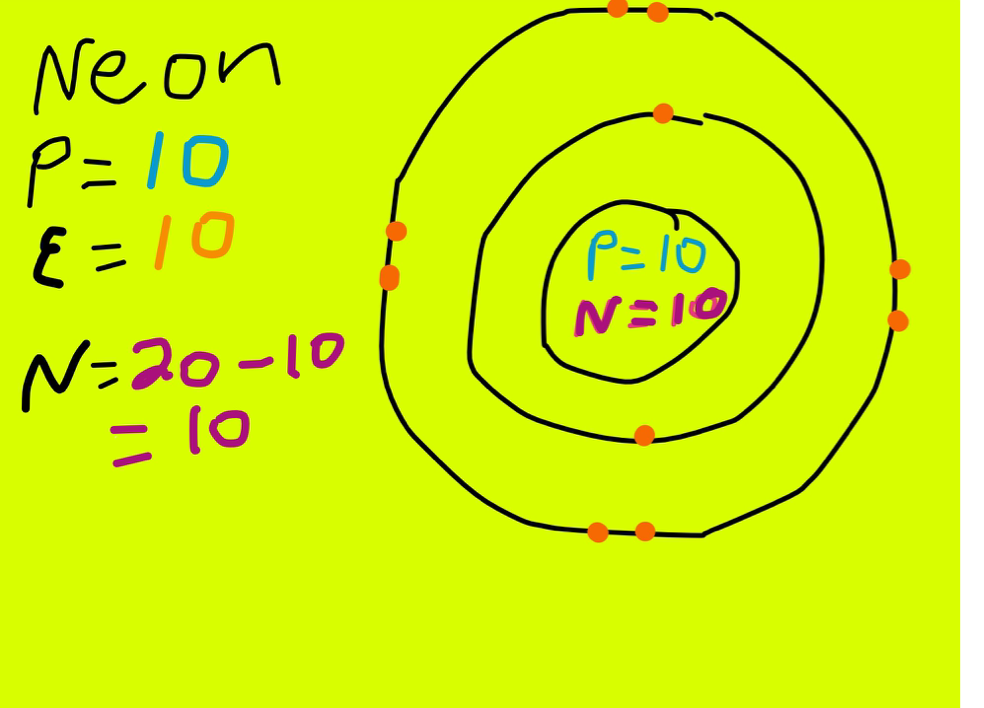
Neon Protons, Neutrons, Electrons Complete Guide
Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.